Optimization of traditional starter culture and African locust beans for daddawa production using Response Surface Methodology (RSM)
DOI:
https://doi.org/10.54117/gjpas.v1i2.28Keywords:
Daddawa, Condiment, Starter Culture, Fermentation, Response Surface MethodAbstract
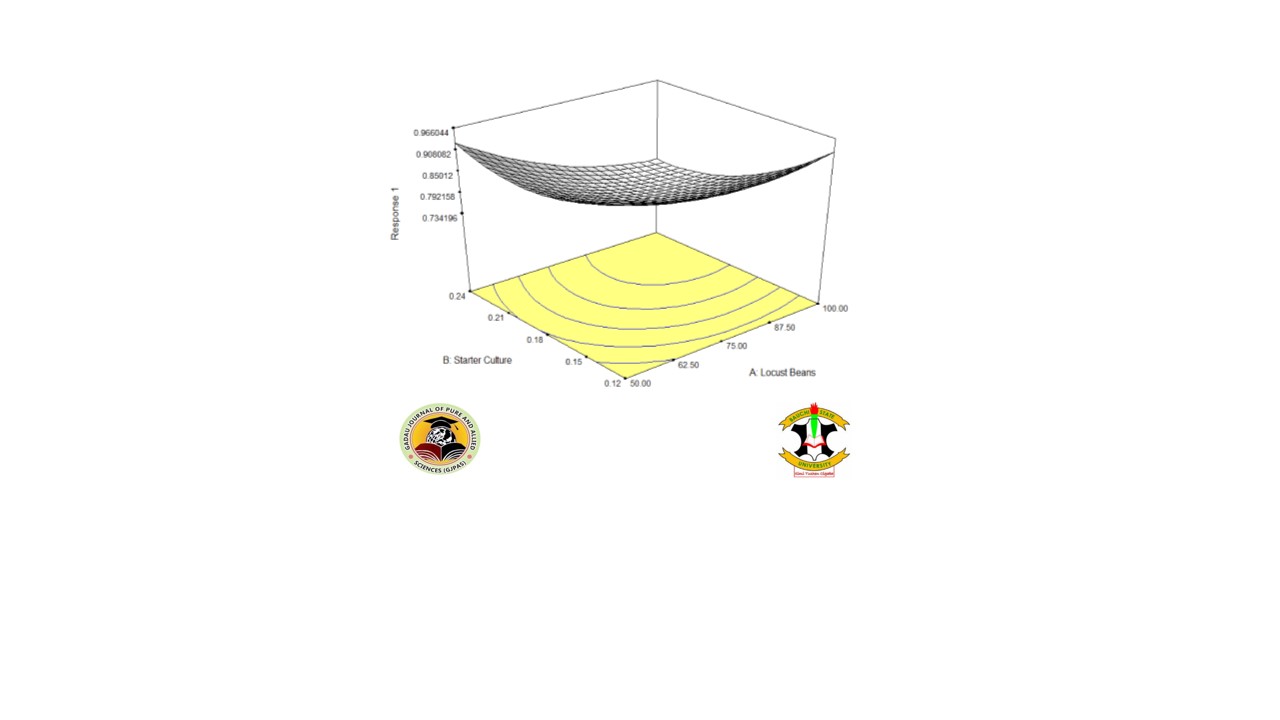
“Daddawa” is a traditional condiment that is produced by the fermentation of African locust beans (Parkia biglobosa). It is a popular food condiment and economical method of food preservation specifically in the West and Central African region. The traditional starter culture used was isolated and identified using biochemical methods. Optimization of fermentation conditions of the starter culture and the locust beans was carried out using a central composite design of response surface methodology (RSM). The fermentation was considered at 40°C for 72 h. Bacteria suspected to be Bacillus subtilis, other Bacillus species, Staphylococcus aureus and other Staphylococcus species were isolated from the traditional starter culture while Bacillus subtilis and other Bacillus species, were further isolated from the optimized product. Locust bean fermentation was found to be optimum at 0.12 g of the starter culture and 50 g of locust beans. The results illustrate 0.994 mg/ml concentration of amino acid (L –Valine) and 8.15 x 106 cfu/ml total mesophilic bacterial count at the optimum parameters. This result revealed that Bacillus species, most importantly Bacillus subtilis are the vibrant bacteria involved in the fermentation process since it was significantly found in the end product.
References
Abdullahi, K.B., Kankara, S.S., Tukur, K., 2020. Effect of Varying Concentrations of Table Salt on the Microbial Fermentation of Parkia biglobosa (Jacq.) Benth Seeds. UMYU Journal of Microbiology Research 5, 98–105.
Achi, O.K., 2005. Traditional fermented protein condiments in Nigeria. African Journal of Biotechnology 4, 1612 - 1621.
Akanni, G.B., 2017. Alkaline fermentation of Bambara groundnut (Vigna subterranean L. Verdc.) as Dawadawa-like African food condiment (PhD Thesis). University of Pretoria.
Antai, S.P., Ibrahim, M.H., 1986. Micro-organisms associated with African locust bean (Parkia filicoidea Welw) fermentation for ‘dawadawa’ production. Journal of Applied Bacteriology 61, 145–148.
Beaumont, M., 2002. Flavouring composition prepared by fermentation with Bacillus spp. International journal of food microbiology 75, 189–196.
Bezerra, M.A., Santelli, R.E., Oliveira, E.P., Villar, L.S., Escaleira, L.A., 2008. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76, 965–977.
Campbell-Platt, G., 2017. Food science and technology. John Wiley & Sons, 2, 1- 576.
Cheesbrough, M., 2006. District laboratory practice in tropical countries, part 2. Cambridge university press.
Farinde, E.O., Adeniran, H.A., Abiose, S.H., 2014. Comparative Microbial Assessment of Fermented Lima Bean (Phaseolus lunatus) and Locust Bean (Parkia biglobossa) in Production of Daddawa. Microbiology Research Journal International 4, 772–784.
Ibrahim, S., Shukor, M.Y., Khalil, K.A., Helmi, M.I.E., Syed, M.A., Ahmad, S.A., 2015. Application of response surface methodology for optimising caffeine-degrading parameters by Leifsonia sp. strain SIU. Journal of Environmental Biology 36, 1215–1221.
Ibrahim, S., Zahri, K.N.M., Convey, P., Khalil, K.A., Gomez-fuentes, C., Zulkarnain, A., Alias, A.S., González-rocha, G., Ahmad, S.A., 2020. Optimisation of biodegradation conditions for waste canola oil by cold-adapted Rhodococcus sp. AQ5-07 from Antarctica. Electronic Journal of Biotechnology 48, 1–12.
Jerry, O.U., 2016. Microbial Technology &Food Security: Microorganisms Put Safe Food on Our Tables, 1, 27 - 674.
Karamba, K.I., Ahmad, S.A., 2019. Mathematical Relationship of Optical Density, Total Viable Count and Microbial Biomass for Growth of Serratia marcescens Strain AQ07 on Cyanide. Journal of Environmental Microbiology and Toxicology 7, 7–9.
Karamba, K.I., Ahmad, S.A., Azham, Z., Syed, M.A., Khalil, K.A., Shamaan, N.A., Dahalan, F.A., Shukor, M.Y., 2016. Optimisation of biodegradation conditions for cyanide removal by Serratia marcescens strain AQ07 using one-factor-at-a-time technique and response surface methodology. Rendiconti Lincei 27, 1–13.
Karamba, K.I., Ahmad, S.A., Zulkharnain, A., Khalid, A., Shukor, M.Y., 2017. Biodegradation of cyanide and evaluation of kinetic models by immobilized cells of Serratia marcescens strain AQ07. Int. J. Environ. Sci. Technol. 14, 1945–1958.
Karamba, K.I., Yakasai, H.M., 2019. Growth Characterization of Bacillus amyloliquefaciens strain KIK-12 on SDS. Journal of Biochemistry, Microbiology and Biotechnology 7, 26–30.
Molinos, A.C., Galvez, A., Raj, A., Chauhan, A., Gupta, A.D., Chye, F.Y., Rapsang, G.F., Abrol, G.S., Bareh, I., Naomichi, I., 2016. Indigenous Fermented Foods of south Asia 7, 1.
Odunfa, S.A., 1985. Biochemical changes in fermenting African locust bean (Parkia biglobosa) during ‘iru’fermentation. International Journal of Food Science & Technology 20, 295–303.
Ogunshe, A.A., Omotosho, M.O., Ayansina, A.D.V., 2007. Microbial studies and biochemical characteristics of controlled fermented afiyo-a Nigerian fermented food condiment from Prosopis africana (Guill and Perr.) Taub. Pakistan Journal of Nutrition 6, 620–627.
Olayinka, E.M., Omobayonle, F., 2010. Evaluation and optimization of critical control points in the production of Iru. Research Journal of Microbiology 5, 1258–1266.
Omafuvbe, B.O., Abiose, S.H., Shonukan, O.O., 2002. Fermentation of soybean (Glycine max) for soy-daddawa production by starter cultures of Bacillus. Food Microbiology 19, 561–566.
Ouoba, L.I.I., Cantor, M.D., Diawara, B., Traore, A.S., Jakobsen, M., 2003. Degradation of African locust bean oil by Bacillus subtilis and Bacillus pumilus isolated from soumbala, a fermented African locust bean condiment. Journal of Applied Microbiology 95, 868–873.
Quansah, L., Mahunu, G.K., Tahir, H.E., Mariod, A.A., 2019. Parkia biglobosa: Phytochemical Constituents, Bioactive Compounds, Traditional and Medicinal Uses, in: Wild Fruits: Composition, Nutritional Value and Products. Springer, 271–284.
Smith, A.M., Agiza, A.H., 1951. The determination of amino-acids colorimetrically by the ninhydrin reaction. Analyst 76, 623–627.
Wafula, E.N., Franz, C.M., Rohn, S., Huch, M., Mathara, J.M., Trierweiler, B., 2016. Fermentation of African indigenous leafy vegetables to lower post-harvest losses, maintain quality and increase product safety. African Journal of Horticultural Science 9, 1–13.
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Gadau Journal of Pure and Allied Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.

