An overview of the general characteristics, pathogenicity, transmission, and diagnosis of human papilloma virus (HPV)
DOI:
https://doi.org/10.54117/gjpas.v1i1.9Keywords:
Human Papilloma Virus, Pathogenesis, Characteristics, Pathogenicity, Transmission, DiagnosisAbstract
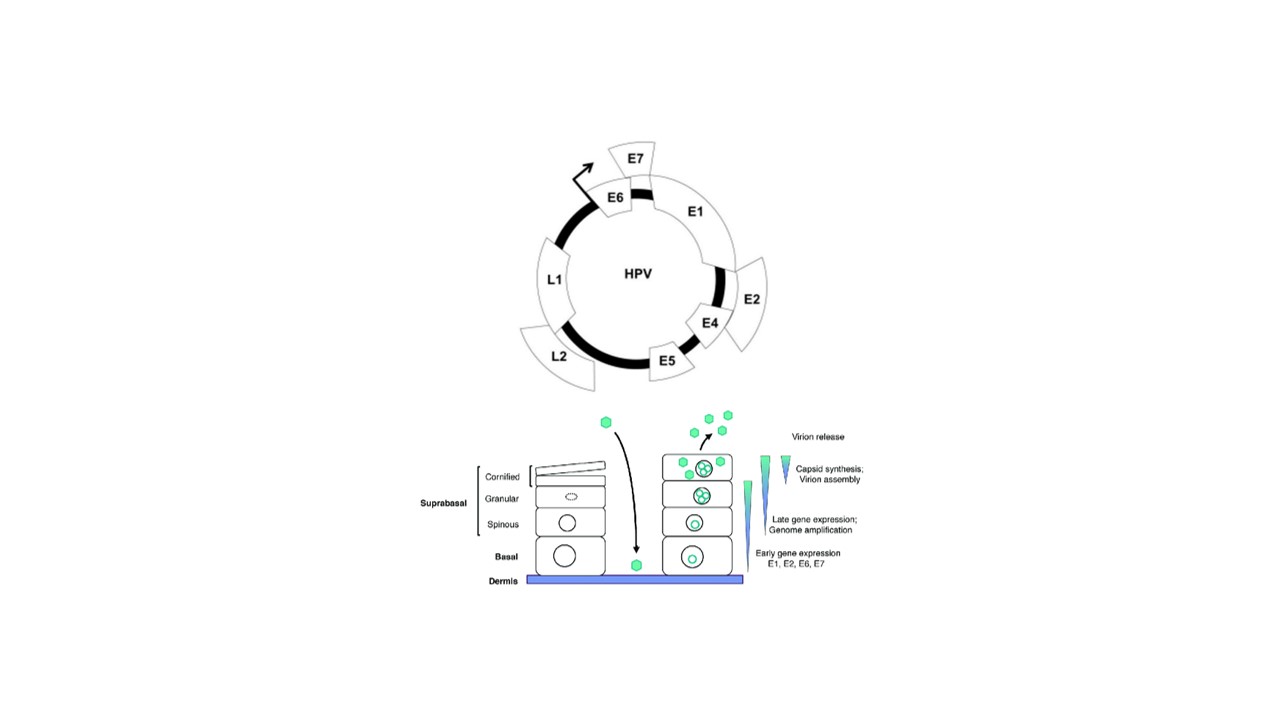
Mucous membrane infections are brought on by the double-standard DNA virus known as human papillomavirus (HPV). Cervix cell alterations are caused by a sexually transmitted illness. E6 and E7 oncogenes play a critical role in HPV infection. Finding these genes to identify HPV strains, particularly the HPV16 strain, will have a significant impact due to its exceptional sensitivity, the dielectric electrochemical biosensor stands out among other pathogen detection methods. Recent evidence suggests that E6 and E7 are also important in inhibiting the innate immune response of the host cell to HPV. Viral replication is mediated by the E1 and E2 proteins in conjunction with other biological stimuli. E2 has also been linked to viral and cellular transcriptional regulation. Despite decades of research, the function of other viral proteins still remains unclear. Therefore, analysis of Human Papilloma Virus (HPV) characteristics, pathogenicity, transmission and diagnosis were reviewed. We concluded that we now have the tools and methodologies necessary to answer this critical question about viral tropism. The discovery of efficient treatments to cure or prevent HPV-induced illness is just as vital as studying the underlying mechanisms of HPV pathogenesis. The identification of drugs specifically to treat HPV infection has not been highly successful due to the complexities of the HPV life cycle and the limited number of enzymatic activities identified for HPV proteins. The development of drug treatments for existing HPV disease is an important undertaking that deserves further attention. In this regard, the development of therapeutic vaccines and self-protection are promising area of investigation and needs to be further supported.
References
Alemany L, Cubilla A, Halec G, Kasamatsu E, Quirós B and Masferrer E, (2016). Role of human papillomavirus in penile carcinomas worldwide. Eur Urol. 69, 953–61.
Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmerón J and Shin HR, (2015). Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 136:98.
Alex Vorsters, Xavier F. Bosch, Mario Poljak, Dur-e-Nayab Waheed, Margaret Stanley and Suzanne M. Garland (2022). HPV prevention and control – The way forward. IPVS Prev Med. 156: 106960.
Andrew Stevenson, Kim Kavanagh, Jiafeng Pan, Lynne Stevenson, Heather Griffin, John Doorbar, Evelyn Scott, Miriam Deeny, Kate Cuschieri and Sheila V Graham (2018) Risk stratification of cervical disease using detection of human papillomavirus (HPV) E4 protein and cellular MCM protein in clinical liquid-based cytology samples Journal of Clinical Virology Volume 108,Pages 19-25
Arbyn, M., Snijders, P.J., Meijer, C.J., Berkhof, J., Cuschieri, K and Kocjan B. J., (2018) Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect21:817–26.
Borgdorf H, Gautam R, Armstrong SD, Xia D, Ndayisaba GF and van Teijlingen NH, (2016). Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol, 9:621–33.
Borgogna, J. C., Shardell, M.D., Santori, E.K., Nelson, T.M., Rath JM., and Glover ED, (2020). The vaginal metabolome and microbiota of cervical HPVpositive and HPV-negative women: a cross-sectional analysis. BJOG, 127(2), 182–92.
Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM and Ravel J, (2014). Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis, 210(11), 1723–33.
Centers for Disease Control and Prevention “CDC” (2020): FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep; 59:626-9.
Chao X, Sun T, Wang S, Tan X, Fan Q and Shi H, (2020). Research of the potential biomarkers in vaginal microbiome for persistent high-risk human papillomavirus infection. Ann Transl Med.;8(4):100.
Cuschieri K, Bhatia R, Cruickshank M, Hillemanns P and Arbyn M. (2016). HPV testing in the context of post-treatment follow up (test of cure). J Clin Virol, 76 Suppl. 1:S56–61.
de Villiers, E.-M. (2004) Relationship between steroid hormone contraceptives and HPV, cervical intraepithelial neoplasia and cervical carcinoma. Int. J. Cancer, 103, 705–708
Di Paola M, Sani C, Clemente AM, Iossa A, Perissi E and Castronovo G, (2017). Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci Rep., 7(1):10200.
Documento de Consenso. Guía de cribado del cáncer de cuello de útero en Espana, (2014). Prog Obstet Ginecol.;57 Suppl. 1:1–20153
Fernandez-Duarte KP, Olaya-Galán NN, Salas-Cárdenas SP, Lopez-Rozo J and Gutierrez-Fernandez MF. (2018). Bifdobacterium adolescentis (DSM 20083) and Lactobacillus casei (Lafti L26-DSL): Probiotics Able to Block the In Vitro Adherence of Rotavirus in MA104 Cells. Probiotics Antimicrob Proteins, 10(1), 56–63.
IARC (2011). Human Papillomaviruses. IARC Library Cataloguing in Publication Data
Ilhan ZE, Laniewski P, Thomas N, Roe DJ, Chase DM and Herbst-Kralovetz MM. (2019). Deciphering the complex interplay between microbiota, HPV, infammation and cancer through cervicovaginal metabolic profling. EBioMedicine, 44:675–90.
Jing, L. S, Gopinath. C.B. Thangavel, L. Yeng Chend, Wong Ruen and Yuane Mei Yangf (2019). Target DNA detection of human papilloma virus-16 E7 gene by capture-target-reporter sandwich on interdigitated electrode sensor. International Journal of Biological Macromolecules, 141, 564-569.
John Doorbar, Wim Quint, Lawrence Banks, Ignacio G Bravo, Mark Stoler, Tom R Broker, Margaret A and Stanley (2016) The biology and life-cycle of human papillomaviruses J.vaccine. PMID: 23199966
Kim MJ, Lee DK, Park JE, Park IH, Seo JG and Ha NJ. (2015). Antiviral activity of Bifdobacterium adolescentis SPM1605 against Coxsackievirus B3. Biotechnol Biotechnol Equip.28(4):681–8.
Klymenko T., Hernandez-Lopez H., MacDonald A. I., Bodily J.M. and Graham S.V. (2016) Human Papillomavirus E2 Regulates SRSF3 (SRp20) To Promote Capsid Protein Expression in Infected Differentiated Keratinocytes Journal of Virology Vol. 90, No. 10
Komatsu A, Igimi S and Kawana K. (2018). Optimization of human papillomavirus (HPV) type 16 E7-expressing lactobacillus-based vaccine for induction of mucosal E7-specifc IFNγ-producing cells. Vaccine.36(24):3423–6.
Li Y, Yu T, Yan H, Li D, Yu T and Yuan T, (2020). Vaginal microbiota and HPV infection: novel mechanistic insights and therapeutic strategies. Infect Drug Resist.13:1213–20.
Lu W, Fang Z, Liu X, Li L, Zhang P, and Zhao J, (2021). The potential role of probiotics in protection against infuenza virus infection in mice. Foods.;10(4):1–16.
Maria, L. M. L., Sonia Pérez-Castrob, Manuel Rodríguez-Iglesiasc, Maria Teresa and Pérez-Graciad (2017). Microbiological diagnosis of human papilloma virus infection. Enferm Infecc Microbiol Clin. 2017;35(9):593–602
Mitra A, Macintyre DA, Marchesi JR, Lee YS, Bennett PR, and Kyrgiou M. (2016). The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome.;4:58.
Moody, C. A. and Laimonis A. L. (2010). Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer, 10(8), 550-60.
Motevaseli E, Azam R, Akrami SM, Mazlomy M, Safari M and Modarressi MH, (2016). The efect of Lactobacillus crispatus and Lactobacillus rhamnosus culture supernatants on expression of autophagy genes and HPV E6 and E7 oncogenes in the HeLa cell line. Cell J.;17(4):601–7.
Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A and Panici PB. (2018). Longterm Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis.18(1):13–9.
Rebecca L Siegel, Kimberly D Miller, Hannah E Fuchs and Ahmedin Jemal (2022). Cancer statistics CA Cancer J Clin: 72(1):7-33. doi: 10.3322/caac.21708
Schwarzer M. (2018). Gut microbiota: puppeteer of the host juvenile growth. Curr Opin Clin Nutr Metab Care, 21(3):179–83.
Sheila V. Graham (2017) The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review J. Clinical science S20160786.
So KA, Yang EJ, Kim NR, Hong SR, Lee JH, and Hwang CS, (2020). Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. PLoS ONE., 15;(9): e0238705.
Terese E. Azua, P. Y. Orka'a and R. I. Ega (2017) Prevalence of Human Papilloma Virus (HPV) Infection among Pregnant Women attending Antenatal Care at General Hospital, Minna – Nigeria Journal of Microbiology and Biotechnology Research216949492
Vriend HJ, Bogaards JA, van Bergen JE, Brink AA, van den Broek IV and Hoebe CJ, (2015). Incidence and persistence of carcinogenic genital human papillomavirus infections in young women with or without Chlamydia trachomatis co-infection. Cancer Med, 4(10),1589–98.
Wei ZT, Chen HL, Wang CF, Yang GL, Han SM and Zhang SL. (2021). Depiction of vaginal microbiota in women with high-risk human papillomavirus infection. Front Public Health, 8:587298.
World Health Organization. Globocan (2012). Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available from: http://globocan.iarc.fr
Yahaya G.M., I. D., Aisha R.S. and Abdullahi B. I (2019) Human Papilloma Virus Infection and Associated Risk Factors Among Pregnant Women Attending Outpient Clinic In General Hospital Lapai, Niger State Nigeria International Journal of Scientific and Research Publications (IJSRP)202797073.
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Gadau Journal of Pure and Allied Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.

