Mathematical model for the transmission dynamics of Leptospirosis in human population
DOI:
https://doi.org/10.54117/gjpas.v2i1.66Keywords:
Leptospirosis, Zoonosis, Model, Stability, Bifurcation, Equilibrium, SimulationAbstract
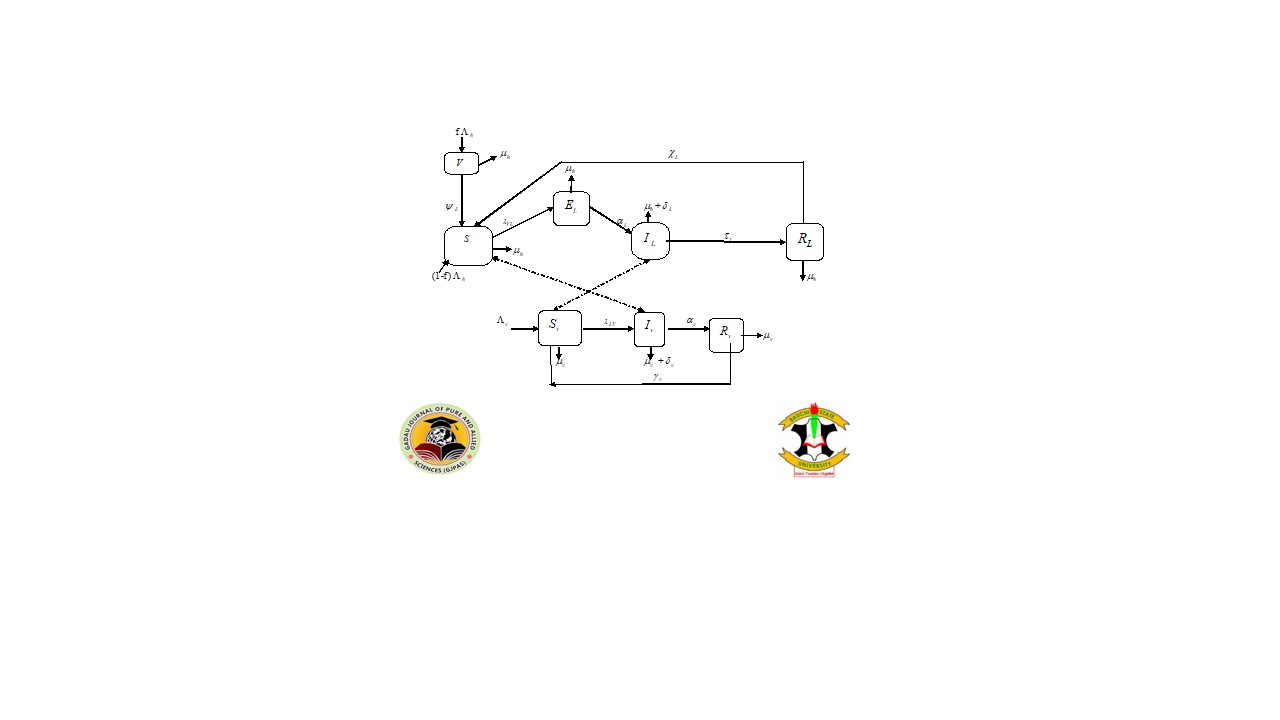
Leptospirosis infection is a contractable disease which is caused by bacteria known as Leptospira. It can lead to serious complications and can pose serious health challenge if not treated promptly and effectively. This paper considered the formulation of a model with eight compartments for leptospirosis transmission in human population to study and analyze the dynamics of the infection. The threshold parameter known as basic reproduction number, a vital quantity for estimating the trends of the spread of the disease was derived. It was discovered from the analysis that model (1) exhibits a disease-free equilibrium point. This was further verified as being both locally and globally asymptotically unchanging whenever the effective basic reproduction number is less than one. Model (1) has an endemic equilibrium point which was as well proved to be both locally and globally asymptotically unchanging whenever the effective basic reproduction number is greater than unity. The study extends its analysis to verify backward bifurcation phenomenon of leptospirosis infection and was ascertained to exist due to loss of temporary immunity as human continue to interact with domestic animals, rodents found in stores and the use of recreational centres such as swimming pool after treatment. Finally, the work suggested the incorporation of vaccination as a control measure to prevent re-infection to human from leptospirosis disease.
References
Albert, I. K.O., Goarant, C. and Picardeau, M. (2009). Leptospira: the dawn of the Molecular Genetics era for an Emerging Zoonotic Pathogen. Nature Review Microbiology, acmillan Publishers Limited, 7. 736 -747.
Allan, K.J. (2016). Leptospirosis in northern Tanzania: Exploring the Role of Rodents and Ruminant Livestock in a Neglected Public Health Problem. PhD Thesis. University of Glasgow, United Kingdom. https://theses.gla.ac.uk.AllanPHD.
Andrawus, J., Nwankwo, A., and Okuonghae, D. (2017), Bifurcation Analysis of a Mathematical Model for TB-dengue Coinfection. Nigerian Research Journal of Engineering and Environmental Sciences. 2(2), 390 -407.
Augusto, F.B., Bewick, S. and Fagan, W.F. (2017). Mathematical Model of Zika Virus with Vertical Transmission. Infectious Disease Modelling. 2, 244-267.
Bharti, A. R., Nally, J.E., Ricaldi, J.N., Matthias, M.A., Diaz, M.M., Lovett, M.A., Levett, P.N., Gilman, R.H., Wiling, M.R., Gotuzzo, E., and Vinetz J.M. (2003). Peru United State Leptospirosis Consortium. Leptospirosis: A Zoonotic Disease of Global importance. Lancet Infect Dis. Dec; 3(12): 757-71.
Castillo-Chavez, C, Song, B., (2004), Dynamical Model of Tuberculosis and their application. Math. Biosc. Eng. 1,361-404.
Eguda, F.Y., Ocheme, C. A., Sule, M. M., and Babura, I. B. (2020), Analysis of a Malaria Transmission Model in Children. African Journal of Environmental Management. 24(5), 789- 798.
Garba, B., Abdul, R. B., Siti, K. B., Zunita, Z., Abdul, R. M. and Faruku, B. (2018). Major Epidemiological Factors associated with Leptospirosis in Malayia. Elsevier Acta Tropica, 178. 242-247.
Gumel, A. B. (2012). Causes of Backward Bifurcation in some Epidemiological Models. Elsevier J. Math. Anal. Appl. 395 (2012) 355 – 365.
LaSalle, J. P. (1967). The Stability of Dynamical System, SIAM, Philadelphia, DOI:10.1016/s1574-6917(07)x0500-2.
Martcheva, M. (2015). An Introduction to Mathematical Epidemiology. Texts in Applied Mathematics, Springer, 61, 1-462. https://link.springer.com
Monahan, A. M., Miller, I. S. and Nally, J. E. (2009). Leptospirosis: Risks during Recreational Activities. Journal of Applied Microbiology 107 (2009), 707-716.
Muhammad, A. K., Saeed, I. and Sher, A. k., (2014). Mathematical Modeling towards the Dynamical Interaction of Leptospirosis. Applied Mathematics and Information Science. An International Journal. 8(3), 1049 -1056.
Muhammad, A.K., Syed, F.S., Saeed, I., Ilyas, K. and Sharidan, S. (2015). Dynamic Behavior of Leptospirosis Disease with Saturated Incidence Rate. Int. J. Appl. Comput. Math. Springer India. 2(4), 435-452.
Pongsumpun, P. (2012). Mathematical Model for the Treatment of Leptospirosis in Juvenile and Adult Human. Internal Journal of Mathematical, Computational, Physical, Electrical and Computer Engineering. 6(12),1639-1644.
Van den Driessche, P., and Watmongh, J. (2002). Reproductive Numbers and Sub-threshold Endemic Equilibria for Compartmental Models of Disease Transmission, Math. Biosci., 80 (2002), 29 -48.
World Health Organization (WHO) (2006), Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. WHO Library Cataloguing-in-publication Data. International Leptospirosis Society. 1-122, https://apps.who.int.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Gadau Journal of Pure and Allied Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.

