Total antioxidant status and adiponectin levels in gestational diabetes mellitus in Kaduna State, Nigeria
DOI:
https://doi.org/10.54117/gjpas.v2i2.110Keywords:
Adiponectin, Antioxidants, Gestational Diabetes Mellitus, Glucose, PregnancyAbstract
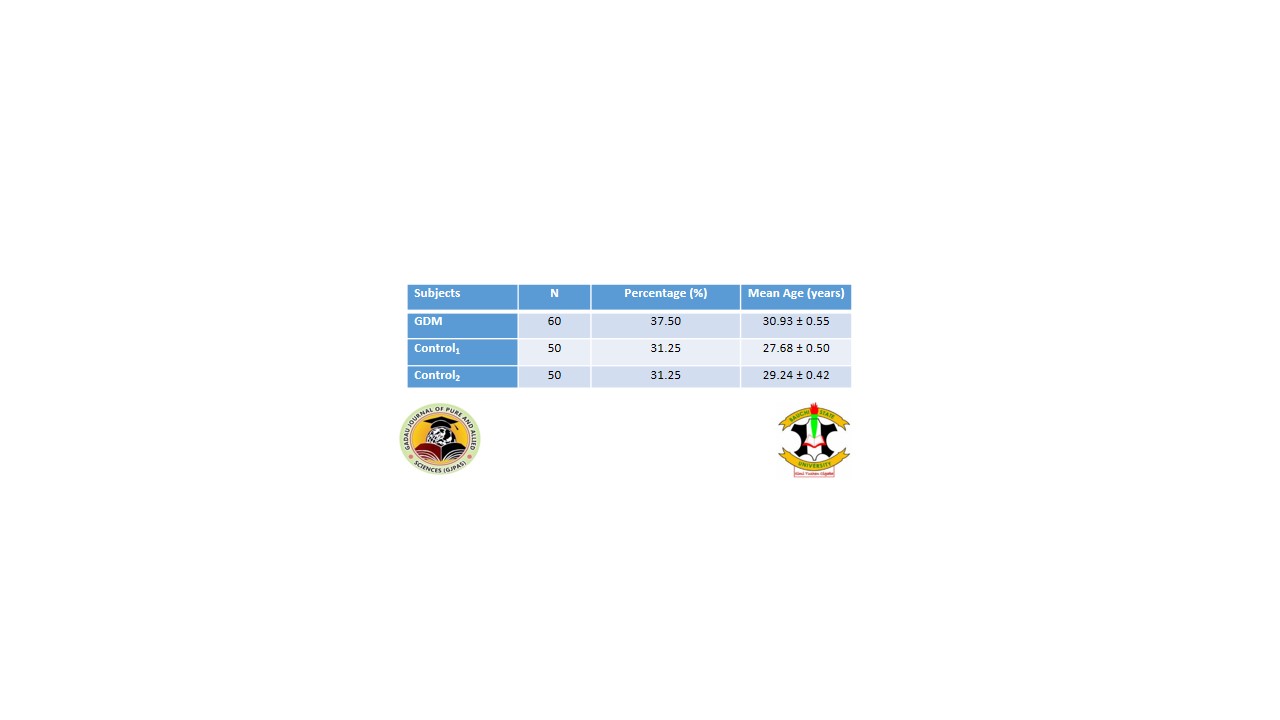
Antioxidants play a key role in treating and reducing the complication of diabetes mellitus. Adiponectin exerts insulin-sensitizing effects and possesses anti-inflammatory and anti-angiogenic properties, which may play crucial roles in gestational diabetes mellitus (GDM) pathophysiology. This study aim at evaluating total antioxidant status and adiponectin levels in gestational diabetes mellitus patients. The study evaluated a cross sectional design in which a total of 160 subjects between the ages of 18- 40 years were recruited for the study. Sixty (60) subjects were gestational diabetic women, 50 non-diabetic pregnant women as Control1 all attending Antenatal clinics at BarauDikko Teaching Hospital, Yusuf Dantsoho Memorial Hospital and GwamnaAwan General Hospital, Kaduna State, Nigeria as well as 50 apparently healthy non-pregnant non diabetic staff as Control2. From the participants, 60 were gestational diabetic mellitus (GDM) (37.50%) with mean age 30.93 ± 0.55years, 50 non GDM pregnant women (31.25%) with mean age 27.68±0.50 years and 50 non GDM non pregnant (31.25%) with mean age 29.24±0.42 years. Adiponectin (APN) level was significantly lower compared with total antioxidant status (TAS) and vitamin C levels in pregnant women. The random blood glucose (RBG), fasting blood glucose (FBG) and 2 hours prospandial blood glucose (2HBG) concentrations in GDM patients and Controls 1 and 2 subjects were all significant different (p < 0.05). However, inter group comparism of C1vsC2 in RBG, FBG and 2HBG were all similar (p>0.05). The correlations between AGE vs BMI was significant (r= 0.276, p < 0.05), Age vs 2HBG (r = -0.256, p <0.05) FBG vsRBG (r = 0.369 p<0.05). FBG versus 2HBG (r = 0.646 p<0.05) and 2HBG vs RBG (r = 0.524, p<0.05) in GDM patients. The study indicated a relationship between total antioxidant capacity (TAS) and adiponectin in pregnant women with GDM compared pregnant women without GDM and control none pregnant women. Majority of the women take daily intake of vegetables, which could positively affect their antioxidant status. There was significantly lower APN compared with TAS, while a significant level of vitamin C compared with APN.
References
Afzal, M., Armstrong, D. (2002). Fractionation of herbal medicine for identifying antioxidant activity. In: Armstrong, D. (Ed.) Methods in Molecular Biology, vol. 186: Oxidative Stress Biomarkers and Antioxidant Protocols, Humana Press Inc.
American Diabetes Association (2018). Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes 2018. Diabetes Care, 41, S13–S27.
Ashcroft, F., Rohm, M., Clark, A., Brereton, M.F. (2017). Is Type 2 Diabetes a Glycogen Storage Disease of Pancreatic β Cells? Cell MeTable 26: 17–23.
Aye IL, et al. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015;112(41): 12858-12863.
Barbour, L., McCurdy, C., Hernandez, T., Kirwan, J.P.,Catalano, P., Friedman, J.E. (2007). Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30: S112–S119.
Bhograj A, Suryanarayana KM, Nayak A, Murthy NS, Dharmalingam M, Kalra P. Serum adiponectin levels in gestational diabetes mellitus. Indian J Endocrinol Metab. 2016 Nov-Dec;20(6):752-755.
Boden G. (2005). Effect of free fatty acids and glucose metabolism: significance for insulin resistance and activate proinflammation nuclear factor-KB Diabetes.1; 54(12): 34 58-65.
Camelo Castillo, W., Boggess, K., Stürmer, T., Brookhart, M.A., Benjamin, D.K., Jonsson, F.M. (2015). Association of Adverse Pregnancy Outcomes with Glyburide vs Insulin in Women with Gestational Diabetes. JAMA Pediatr. 169: 452–458.
Catalano, P.M. (2014). Trying to understand gestational diabetes. Diabet. Med. 31: 273–281.
decompensation during the progression of diabetes. Diabetes 50 : S154–159.
DeFronzo, R.A. (2009). From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes, 58:773–795.
Durnwald CP, et al. Adiponectin levels in women with a history of gestational diabetes mellitus. Ann Intern Med. 2006;144(5): 328-332.
Feig, D., Moses, R.G. (2001). Metformin Therapy during Pregnancy Good for the goose and good for the gosling too? Diabetes Care 34: 2329–2330.
Ferrara A. (2007). Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. ;30(Suppl 2):S141–S146. [PubMed] [Google Scholar]
Friedman, J., Kirwan, J., Jing, M., Presley, L., Catalano, P.M. (2008). Increased Skeletal Muscle Tumor Necrosis Factor-α and Impaired Insulin Signaling Persist in Obese Women with Gestational Diabetes Mellitus 1 Year Postpartum. Diabetes 57: 606–613.
Halliwell, B., Aeschbach, R., Loliger, J., Aruoma, O.I. (1995). The characterization of antioxidant. Food and Chemical Toxicology 33(7): 601–617
Homko, C., Sivan, E., Chen, X., Reece, E., Boden, G. (2001). Insulin secretion during and after
International Diabetes Federation. (2017). IDF Diabetes Atlas, 8th edition.; IDF: Brussels, Belgium.
Muhas C, Naseef PP. A review article-gestational diabetes mellitus. Int J Curr Pharm Res 2017;9(1):1-5.
Plows, J., Stanley, J., Baker, P., Reynolds, C., Vickers, M.H. (2018). The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 19 3342.
Prentki, M., Nolan, C.J. (2006). Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 116: 1802–1812.
Retnakaran R, et al. Adiponectin and β cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005;48(5): 993-1001.
Surapaneni KM, Vishnu PV. Antioxidant enzymes and vitamins in gestational diabetes. J Clin Diagn Res. 2008;2:1081–1085.
Weir, G., Laybutt, D., Kaneto, H., Bonner-Weir, S., Sharma, A. (2001). Beta-cell adaptation and pregnancy in patients with gestational diabetes mellitus. J. Clin. Endocrinol. MeTable 86: 568–573.
Worda, C., Leipold, H., Gruber, C., Kautzky-Willer, A., Knöfler, M., and Bancher-Todesca, D. (2004). Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. American journal of obstetrics and gynecology, 191(6), 2120–2124.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Gadau Journal of Pure and Allied Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.

